The Kidney Disease: Improving Global Outcomes (KDIGO) committee has updated their 2012 guidelines for the management of anemia in patients…
 Policy and Guidelines
Policy and Guidelines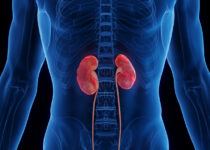





Copyright © 2026 John Wiley & Sons, Inc. All Rights Reserved.
Privacy Policy
