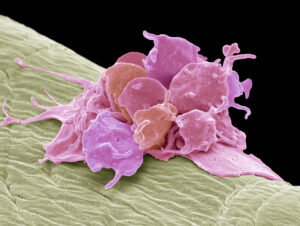
Both heparin-induced thrombocytopenia (HIT) and vaccine-induced immune thrombotic thrombocytopenia (VITT) are rare but serious drug reactions. HIT and VITT are caused by the formation of abnormal antibodies to platelet factor 4 (PF4), which activates platelets to release thrombospondin-1 (TSP1). Currently, diagnosis of HIT and VITT relies on sensitive but nonspecific ELISAs, which must be confirmed with complex functional tests in specialized reference laboratories causing delays in diagnosis and excessive use of costly non-heparin anticoagulants. To simplify the testing process, researchers have investigated the use of cryopreserved platelets coupled with a novel ELISA-based TSP1 end point. Briefly, carefully cryopreserved platelets (which were stored up to one year) were treated with PF4 or heparin and then incubated with serum from patients with suspected HIT or VITT. TSP1 from the collected supernatants was then measured by ELISA. To determine the diagnostic accuracy of this new assay, 34 cases of suspected HIT, which had already been confirmed positive or negative by functional tests were used. The serum from patients with confirmed HIT (n=16) induced a mean-fold increase of 2.67 (95% C.I., 2.44 to 2.90) of TSP1 compared to the serum from patients with suspected HIT (n=18) who lacked platelet-activating antibodies. As expected, four VITT samples did not activate cryopreserved platelets in the presence of heparin, only PF4. Further refinement of this streamlined diagnostic assay using cryopreserved platelets is needed.
Reference: