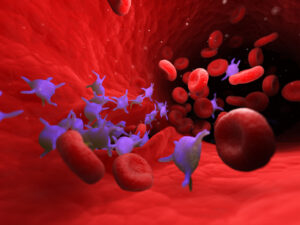
Platelet-RBC (P-RBC) complexes are associated with several diseases such as sickle cell disease, malaria, and immune thrombocytopenia. Since diseased RBCs exhibit similar morphology to aged and senescent RBCs, and P-RBC complexes have been observed in up to 0.3% of human RBCs in healthy individuals, researchers hypothesized that the P-RBCs may form in order to be targeted for clearance from circulation when undergoing senescence. Using a pulse-chase mouse model, researchers labeled RBCs and tracked them over 60 days (the lifespan of murine RBCs) and detected ten-fold more P-RBC complexes in 50 to 60 day-old RBCs compared to those less than 50 days. P-RBC complexes were removed seven-times faster than RBCs and three-times faster than platelets via erythrophagocytosis in the spleen, and platelets were required to maintain senescent RBC clearance. Corollary studies in human patients (n=16) showed higher levels of P-RBC complexes in the bloodstream 60 days post-splenectomy compared to healthy controls. Furthermore, nine patients with immune thrombocytopenia had decreased P-RBC complexes and increased aged RBCs compared to healthy controls. Future research is needed to investigate how this novel pathway is involved in disease pathogenesis.
References: