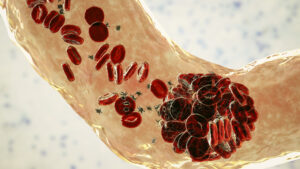
Congenital thrombotic thrombocytopenic purpura (TTP) is a rare disease caused by a severe hereditary deficiency of ADAMTS13, which normally cleaves von Willibrand factor (VWF). With the accumulation of multimers of VWF with high platelet-binding activity, congenital TTP often manifests as thrombocytopenia since platelets are consumed in forming micro-clots, which may lead to organ failure or even death. Standard treatment involves ADAMTS13 replacement from donor-derived plasma and/or plasma products. In November 2023, recombinant ADAMTS13 (rADAMTS13) was approved by the US Food and Drug Administration for congenital TTP treatment; an encouraging interim analysis from a phase 3, multinational, open-label, crossover trial of rADAMTS13 treatment was recently published. Briefly, 48 adults and children with congenital TTP were randomized 1:1 to receive either rADAMTS13 or standard therapy as prophylaxis for the first 6-month block; in the second 6-month block, the randomization blocks were switched (i.e. crossed-over); and in the third 6-month block, all patients received rADAMTS13. None of the patients receiving rADAMTS13 had an acute TTP event compared to one event in a patient receiving standard therapy. On average, ADAMTS13 activity returned to 100% normal levels under rADAMTS13 therapy compared to 19% with standard therapy. In addition, 9% of patients experienced mild or moderate adverse events while receiving rADAMTS13 compared to 48% with standard therapy. The risk of immunogenicity appears to be low, but 14 patients are continuing in the trial to continue to monitor the safety and efficacy of rADAMTS13.
Reference: