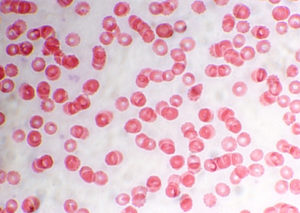
Babesia microti is a tick-borne pathogen responsible for babesiosis—a malaria-like hemolytic disease. Currently, there is not a licensed screening test for B. microti, which is responsible for the most fatalities due to parasitic transfusion-transmitted infections. As reported in The New England Journal of Medicine, researchers at the Red Cross recently investigated a screening protocol for B. microti. Arrayed fluorescence immunoassays (AFIAs) and real-time PCR assays were performed on donated blood samples to determine antibody reactivity and parasite loads. Over 89,000 blood donations were screened from 4 endemic states (Minnesota, Wisconsin, Massachusetts, and Connecticut); 335 (0.38%) were positive for B. microti, and at least one third of these samples were considered infectious based on animal studies. However, no cases of babesiosis were transmitted via transfusion from screened samples to patients in the study. During the two year study period, 29 cases of transfusion-transmitted babesiosis were reported from unscreened samples. The estimated risk of transfusion-transmission in endemic states was 1 case per 101,000 donations. Screening for both antibodies to B. microti and parasite DNA may help to reduce the risk of transfusion-transmitted B. microti infections.
Reference: