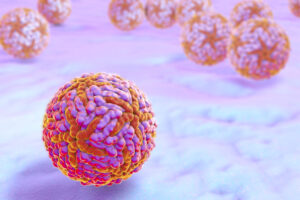
In 2015 and 2016, large outbreaks of the mosquito-borne Zika virus occurred in the Americas. Infection with the Zika virus is usually asymptomatic or very mild. However, infection during pregnancy can cause microcephaly and has also been linked to Guillain-Barré syndrome. In 2016, local transmission of the virus in Texas and Florida prompted the U.S. Food and Drug Administration (FDA) to recommend screening blood donors for the Zika virus. Cases peaked in 2016, and no confirmed cases of local transmission have occurred in the continental U.S. or its territories since 2017. While the FDA will continue to monitor the epidemiology of the Zika virus, it does not currently consider the Zika virus a “relevant communicable disease agent or disease.” Thus, the FDA has withdrawn the May 2018 guidance titled, “Donor Screening Recommendations to Reduce the Risk of Transmission of Zika Virus by Human Cells, Tissues, and Cellular Tissue-Based Products,” and donor screening for Zika virus is not currently needed. A change in the epidemiology of the Zika virus may prompt the FDA to reinstate measures to reduce transmission.
Reference: